A Molecule that can Store the Sun’s Energy for 18 Years
 Molecular solar?
Molecular solar?
The development of solar energy can potentially meet the growing requirements for a global energy system beyond fossil fuels. But a key necessity to unlock the true potential of solar and for that matter renewable energy sources is the development of new and scalable technologies for energy storage.
And now a team of scientists and researchers from Sweden have revealed a breakthrough molecule in solar thermal fuel, which they claim can store the sun’s energy for a period of 18 years.
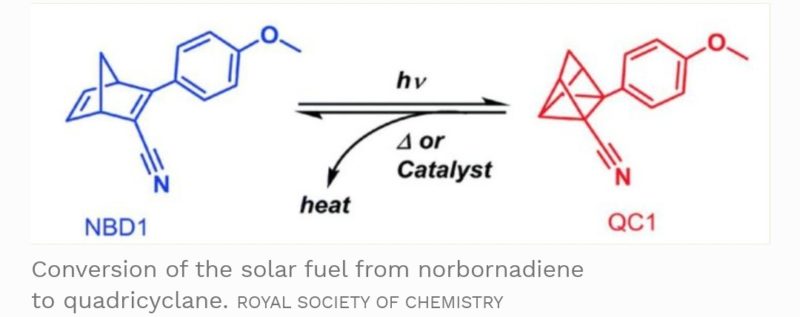
The fuel is composed of carbon, hydrogen and nitrogen molecules. The original structure of the fuel source is based on being norbornadiene molecules. But, when these molecules interact with sunlight, some of the bonds between the atoms are rearranged to form quadricyclane.
This chemical conversion into a quadricyclane molecular structure referred to as an isomer, traps energy within the molecule with strong chemical bonds. Strong enough to hold on to the energy for nearly two decades unless activated.
As batteries continue to develop in their capacity to store more energy for longer periods of time, they have superseded hydrocarbons as a solid source of energy for power on demand. A knowledgeable example of that is vast advancements in electric vehicles that we’re witnessing today.
As an alternative to conventional batteries like the Lead acid, Lithium-ion or even the concept Zinc-air batteries, the specialized solar thermal fluid can hold the sun’s energy for very long periods of time and efficiently discharge that energy in the form of heat on demand. Unlike batteries, which discharge electricity, the solar thermal fuel emits heat when activated through a catalyst. This means the fluid would be ideal for heating residential and commercial homes.
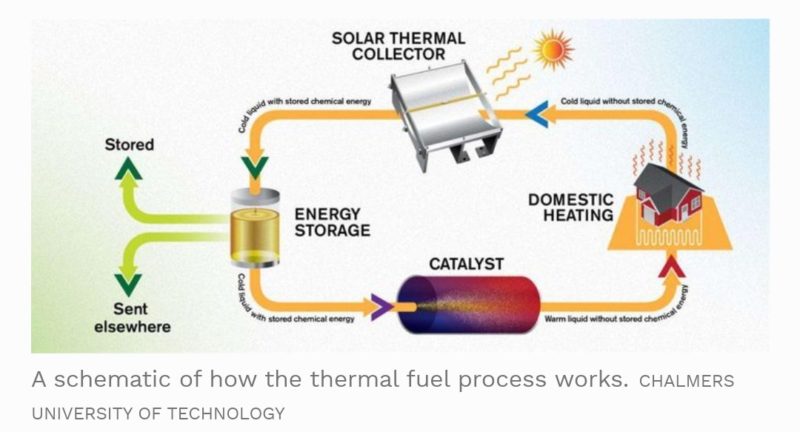
The team found that the catalyst process which rearranges the atoms to form norbornadiene, heats up the fuel by 63 degrees Celsius (113 degrees Fahrenheit). This means if the ambient temperature in the room is around 70 degrees Fahrenheit, the fluid would heat up to 183 degrees F. The heated fluid could then be used to heat homes, commercial buildings, etc. With additional testing and optimization, the team believes they can produce a molecule that can heat up the fuel by over 176 degrees F. The fuel could then be considered for electricity generation.
Requiring no external source of energy to operate except sunlight, and always working in a closed loop. The thermal fuel represents a future with a wider and much greater acceptance of renewable energy coupled with viable and economically sustainable energy storage solutions.
Link to paper – https://pubs.rsc.org/en/content/articlehtml/2018/ee/c8ee01011k




