Can Water be the answer for Battery Storage?
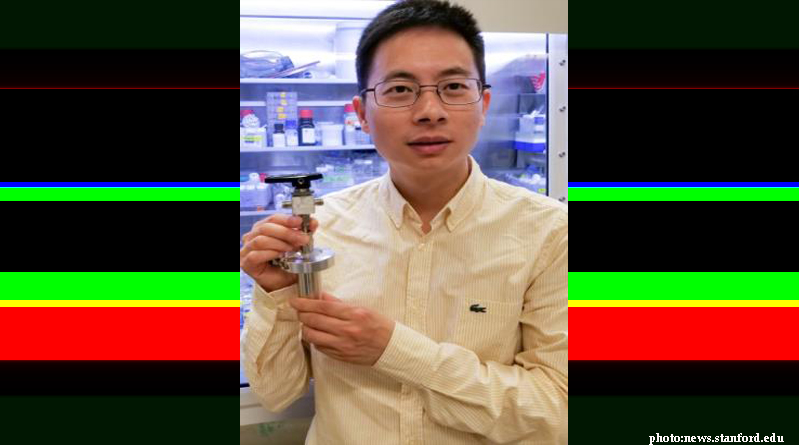
Researchers at Stanford University have developed a water-based battery, which could be a cheaper alternative to store wind or solar energy, so as to feed energy back into the grid and redistribute it when the demand is high.
The manganese-hydrogen ion battery is said to overcome the drawbacks faced by lithium-ion batteries regarding cost, life cycle and cooling systems.
The team led by Professor Yi Cui, is confident that the battery that stands three inches tall and generates a mere 20 milli-watt per hour of power has the ability to withstand more charge/discharge cycles than a lithium-ion battery at a considerably lower price.
The team estimates given the battery’s expected life span, that it would cost a penny to store enough electricity to power a 100W light bulb for 12 hours.
“We believe this prototype technology will be able to meet Department of Energy (DOE) goals for utility scale electrical storage practicality,” said Cui in a statement released by the research team.
The team coaxed a reversible electron-exchange between water and manganese sulfate (a cheap but abundant industrial salt). The researchers then attached a power source to the prototype in order to mimic the feed of power (wind or solar) into it. This causes the electrons flowing in to react with the manganese sulfate and leaves particles of manganese dioxide clinging to the electrodes. The excess electrons bubble off in the form of hydrogen gas. For the next step the team reattached their power source to a depleted prototype in order to induce the manganese dioxide particles clinging to the electrodes to mix with the water and replenish the manganese sulfate salt. Once the salt is restored, the incoming surpluses of electrons bubble of as hydrogen gas. The process can be repeated again and again.
“What we’ve done is thrown a special salt into water, dropped in an electrode, and created a reversible chemical reaction that stores electrons in the form of hydrogen gas.” Said Cui.
The battery is still just a prototype and is currently being further developed. The prototype uses platinum as a catalyst to spur the chemical reactions at the electrodes in order to make it efficient. This would cause the cost to be prohibitive on a large production scale. However, the team claims to have indentified alternative catalysts that could bring down the cost to $100 per kilowatt-hour.
The team is currently doing 10000 recharges of the prototype (double the DOE requirements) but is yet to test the manganese-hydrogen ion battery under actual storage grid conditions in order to truly asses its cost and lifetime performance.
Former Department of Energy (DOE) secretary and Nobel Laureate Steven Chu said, “While the precise materials and design still need development, this prototype demonstrates the type of science and engineering that suggest new ways to achieve low cost, long lasting, utility scale batteries.”
The team aims to develop the battery on an industrial scale that would satisfy the target set by the DOE. As per the DOE recommendations, a battery for grid-scale storage should be able to store and discharge a minimum of 20 kilowatt per hour of energy and be capable to withstanding at least 5000 recharges. Furthermore, the battery should have a lifespan of minimum 10 years and should cost a total of $20,000 or less, or $100 per kilowatt-hour.
If the prototype works under grid-scale conditions, then its novel chemistry, low-cost materials and relative simplicity could make it the ideal low-cost storage option.
![]()