Research Shows Calcium Instead of Lithium Can Make Better RE Batteries

The advent of renewable energy with its innate unpredictable nature has brought a global need for effective energy storage. This need has so far fuelled the demand for Lithium which can easily be stored, transported to the solar and wind power sites and work when any one of the sources is not. But the availability of the metal has its problem. Majority of these metals are mined from Congo or China which hurts the environment and the countries pockets who are trying to decarbonize their grids.
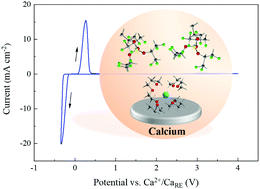
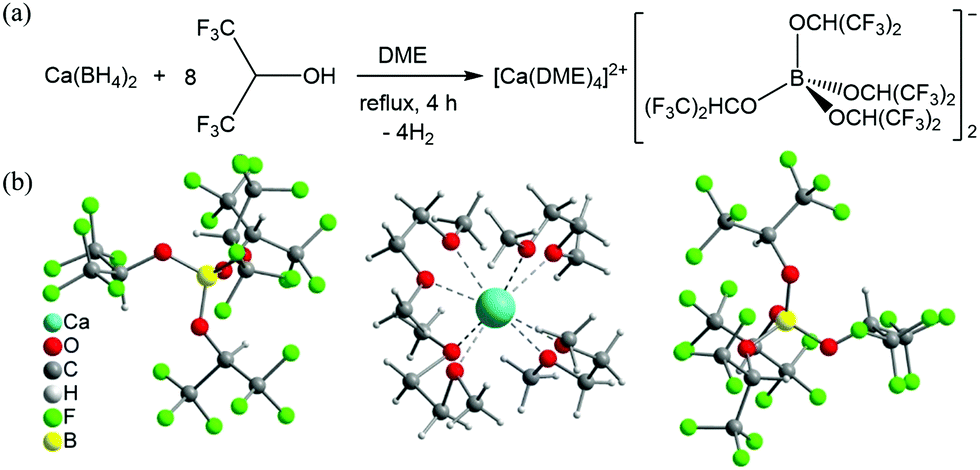
The scientists all over the world have been interested in finding the next technology that could follow Lithium batteries and compete in terms of rechargeability and cost. One such progress has been made by German researchers of Helmholtz Institute Ulm (HIU) Electrochemical Energy Storage. The research has come up with a solution for Calcium batteries. Calcium is already being used in Lead-acid batteries however, Calcium battery (Ca) development has been hampered due to lack of efficient electrolyte. The German researchers have formulated a novel calcium tetrakis (hexafluoro isopropyl oxy) borate Ca[B(hfip)4]2 based electrolytes with a wide electrochemical window and good chemical stability, which may pave the way for high-energy Calcium batteries.
The electrolyte exhibits reversible Ca deposition at room temperature, high oxidative stability up to 4.5 V and high ionic conductivity >8 mS cm−1. This finding opens a new approach to room-temperature rechargeable calcium batteries.
According to the research, development of multivalent battery technologies like magnesium (Mg) and calcium (Ca) anodes that are based on elements that are more abundant is considered to be a promising option to achieve improved energy densities and at the same time significantly reduce cost.
Multivalent ions are capable of transferring double or triple electrons per ion, thus offering the promise of a two- or three-fold increase in the capacity as compared to the monovalent Li-ion and Na-ion batteries.
Unlike the raw materials such as cobalt (Co), nickel (Ni) and lithium (Li), which are essential components in commercial Li-Ion Batteries, Calcium is the fifth most abundant element in the earth’s crust with equal geographical resource distribution and has the advantages of safety, nontoxicity and low cost.
The researchers at Helmholtz Institute Ulm in Germany used a salt as an electrolyte here. More specifically, they “reacted a calcium compound with a fluorine-containing compound to create a new type of calcium salt,” according to a description in Nature.
The researchers do add that there’s still more progress needed, with the team saying that “further improvement will be carried out by modifying the electrolyte composition e.g. solvent, concentration and additive.” Now that an electrolyte has been discovered, in other words, it can be improved.
The scientific journal does add that such developments mean that Calcium-based batteries could be used in industrial-scale systems to store wind and solar energy in the future.