A New Prospect For Renewable Energy Storage: Proton Conducting Fuel Cell

Alternative sources of energy are being widely explored and accepted across the world where viable. And, there is now a crucial need for making renewable energy a reality by having access to technologies that can convert electricity from wind and sun and renewable sources into chemical forms for storage over long durations and then back to electricity when required. The existing commercial devices that do the job are mostly very costly and are not fully efficient in doing both the jobs. But researchers have now developed lab-scale devices that could perform both the jobs.
As a way out, many utilities are installing massive banks of batteries, take for instance Tesla’s massive battery plant in Australia. However, not only are these batteries expensive, but they also store the energy to back up the grid only for a few hours.
Another option comes in the way of converting the energy into hydrogen fuel. There are devices—electrolysers that do this task by using electricity from wind and solar power. They split water into oxygen and hydrogen gas, which is a carbon-free fuel. The second type of device called the ‘fuel cells’ can convert the hydrogen back into electricity that could power cars, trucks, buses or even feed the power grids.
But this method is not free of problems. Commercial electrolysers and fuel cells use different catalysts to speed up the two reactions, leaving both devices useless if being operated without the other. To address this problem, researchers have been trying a new type of fuel cell—the proton conducting fuel cell (PCFC).
What are PCFCs?
The PCFC can make fuel and reconvert it to electricity using just one set of catalyst.
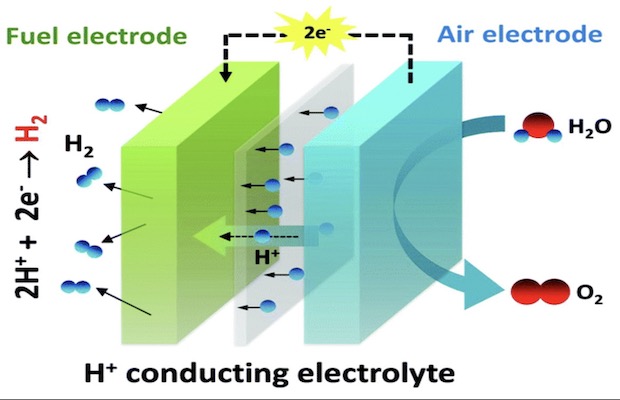
The PCFCs consist of two electrodes separated by a membrane that allows the proton across. The first electrode known as the air electrode has steam and electricity that are fed into a ceramic catalyst which splits the steam’s water into positively charged hydrogen ions, oxygen molecules and electrons. The electrons generated travel through an external wire to the second electrode—the fuel electrode. It is the fuel electrode where the electrons meet up the protons that cross through the membrane. Here, a nickel-based catalyst combines them to make hydrogen gas. In previous PCFCs, the nickel catalyst was found to perform fine, but the ceramic catalyst was inefficient. Much of the electric energy was lost as heat in it.
And now, two research teams have worked out the way for a better performance by the ceramic catalysts.
Researchers led by chemist Sossina Haile of Kumoh National Institute of Technology, Korea working at Northwest University reported in their paper published in the journal Energy & Environmental Science, that they developed a fuel electrode made from a ceramic alloy that contains six elements and harnessed 76% of its electricity to split water molecules.
The other team, however, reported that they managed to develop a ceramic alloy electrode made up of five elements, which harnessed as much as 98% of the energy it was fed to split water molecules.
The team led by chemist Ryan O’hayre at the Colorado School of Mines, published their paper in the journal Nature Energy, and revealed that they used 11 different fuels – hydrogen, methane, domestic natural gas (with and without hydrogen sulphide), propane, n-butane, i-butane, iso-octane, methanol, ethanol, and ammonia – demonstrating excellent performance and exceptional durability across all types over thousands of hours of operation.
Adding that the new cells would also work in reverse as electrolysers, efficiently breaking down water into hydrogen and oxygen.

“In this way, our reversible fuel cell device can act as a battery,” said Ryan O’Hayre, professor of metallurgical and materials engineering and co-lead author of the paper with postdoctoral researcher Chuancheng Duan. “We can use our device to make hydrogen from water when there is excess renewable electricity on the grid that might otherwise go to waste. The hydrogen can be stored for later use or used in the chemical industry for various purposes. If we store some or all of the hydrogen, we can then also run our device in fuel cell mode using this stored hydrogen to produce electricity at times when there is not enough electricity available on the grid.”
If these lab-scale devices can be made on a larger scale, it can contribute greatly to making renewable energy more affordable and also help in realising the dream of running the world on renewables.




